Standard Enthalpy Of Formation Is Zero For Sulphur . for any element at any particular temperature, we define the standard enthalpy of formation to be zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of formation for an element in its standard state is zero!!!! For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. the standard enthalpy of formation of any element in its standard state is zero by definition. Elements in their standard state are not. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,.
from www.studeersnel.nl
the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Elements in their standard state are not. for any element at any particular temperature, we define the standard enthalpy of formation to be zero. the standard enthalpy of formation for an element in its standard state is zero!!!! the standard enthalpy of formation of any element in its standard state is zero by definition.
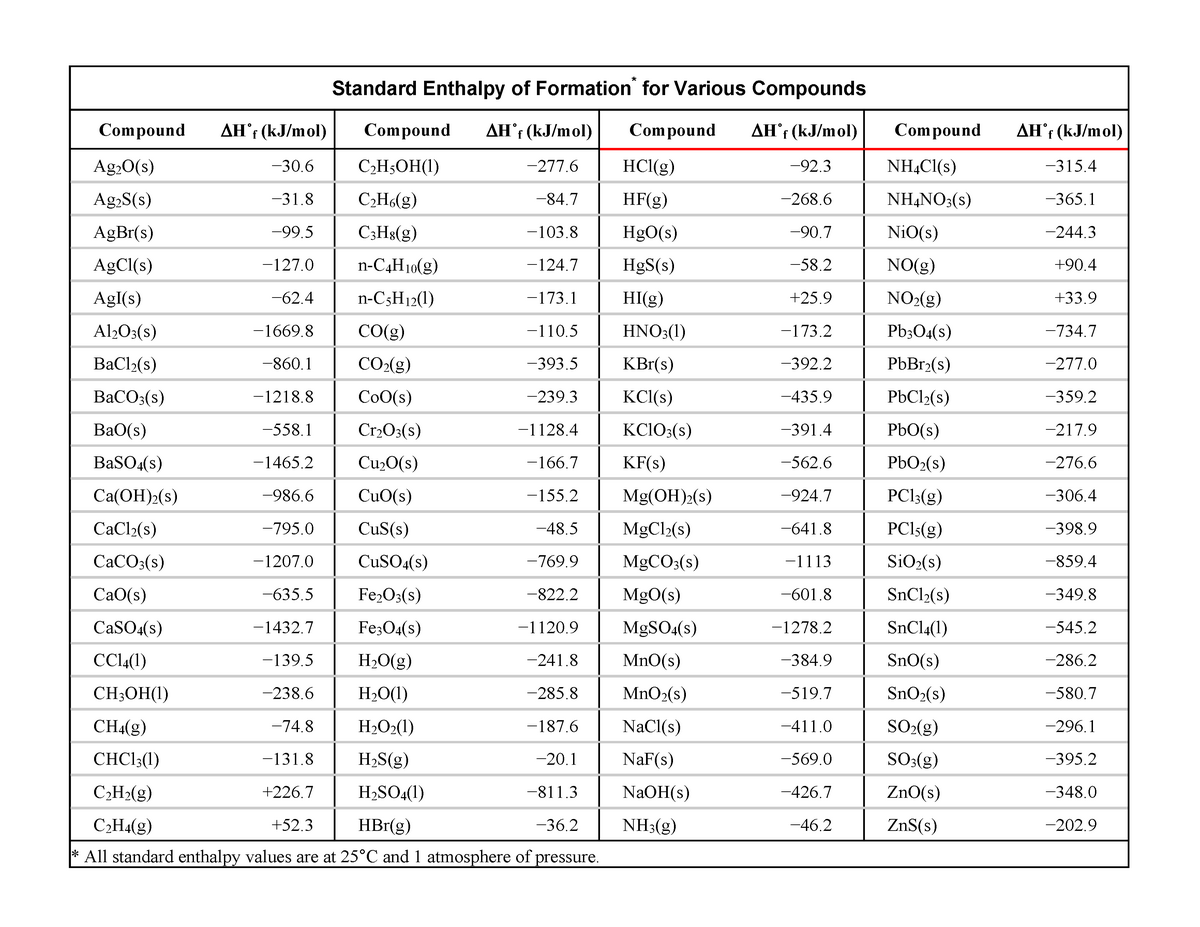
Rstandard enthalpy of formation Table useful Standard Enthalpy of
Standard Enthalpy Of Formation Is Zero For Sulphur by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. Elements in their standard state are not. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation for an element in its standard state is zero!!!! the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. for any element at any particular temperature, we define the standard enthalpy of formation to be zero. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the.
From www.chegg.com
Solved The standard enthalpies of formation for several Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation of any element in its standard state is zero by definition. Elements in their standard state are not. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. by definition, the standard enthalpy of formation. Standard Enthalpy Of Formation Is Zero For Sulphur.
From www.numerade.com
SOLVEDThe standard enthalpies of formation for S(g), F(g), SF4(g), and Standard Enthalpy Of Formation Is Zero For Sulphur 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation for an element in its standard state is zero!!!! by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,.. Standard Enthalpy Of Formation Is Zero For Sulphur.
From www.studeersnel.nl
Rstandard enthalpy of formation Table useful Standard Enthalpy of Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Is Zero For Sulphur.
From kunduz.com
[ANSWERED] Use the standard enthalpies of formation to calculate the AH Standard Enthalpy Of Formation Is Zero For Sulphur by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. the standard enthalpy of formation for an element in its standard state is zero!!!! Elements in their standard state are not. the standard enthalpy of formation is a measure of the energy released or consumed. Standard Enthalpy Of Formation Is Zero For Sulphur.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation for an element in its standard state is zero!!!! for any element at any particular temperature, we define the standard enthalpy of formation to be zero. 193 rows in. Standard Enthalpy Of Formation Is Zero For Sulphur.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Is Zero For Sulphur 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. for any element at any particular temperature, we define the standard enthalpy of formation to be zero. the standard enthalpy of formation for an element in its standard state is zero!!!! the standard enthalpy of. Standard Enthalpy Of Formation Is Zero For Sulphur.
From dshfembfeco.blob.core.windows.net
Standard Enthalpy Of Formation So2 at Betty Joseph blog Standard Enthalpy Of Formation Is Zero For Sulphur by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. Elements in their standard state are not. the. Standard Enthalpy Of Formation Is Zero For Sulphur.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. Elements in their standard state are not. for. Standard Enthalpy Of Formation Is Zero For Sulphur.
From dxouglhlh.blob.core.windows.net
Standard Enthalpy Of Formation Mg at David Leon blog Standard Enthalpy Of Formation Is Zero For Sulphur For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. Elements in their standard state are not. for any element at any particular temperature, we define the standard. Standard Enthalpy Of Formation Is Zero For Sulphur.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Enthalpy Of Formation Is Zero For Sulphur for any element at any particular temperature, we define the standard enthalpy of formation to be zero. the standard enthalpy of formation for an element in its standard state is zero!!!! For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Elements in their standard state are not. by definition, the standard enthalpy of. Standard Enthalpy Of Formation Is Zero For Sulphur.
From exokycsnc.blob.core.windows.net
Standard Enthalpy Of Formation Table Elements at Filomena Gilbert blog Standard Enthalpy Of Formation Is Zero For Sulphur by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. . Standard Enthalpy Of Formation Is Zero For Sulphur.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of formation for an element in its standard state is zero!!!! by definition, the standard enthalpy of formation of an element in its most stable form is. Standard Enthalpy Of Formation Is Zero For Sulphur.
From byjus.com
When 0.5 g of sulphur is burnt to SO_2 ; 4.6 kJ of heat is liberated Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation for an element in its standard state is zero!!!! the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. by definition, the standard enthalpy of formation of an element in its most stable form is. Standard Enthalpy Of Formation Is Zero For Sulphur.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation of any element in its standard state is zero by definition. for any element at any particular temperature, we define the standard enthalpy of formation to be zero. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Enthalpy Of Formation Is Zero For Sulphur.
From ceuuptlx.blob.core.windows.net
Standard Enthalpy Of Formation To Zero at Deanne Fox blog Standard Enthalpy Of Formation Is Zero For Sulphur 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of formation is a measure of. Standard Enthalpy Of Formation Is Zero For Sulphur.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. the standard enthalpy of formation, also known as the heat of formation, is the. Standard Enthalpy Of Formation Is Zero For Sulphur.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Is Zero For Sulphur the standard enthalpy of formation for an element in its standard state is zero!!!! by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. for any element at any particular temperature, we. Standard Enthalpy Of Formation Is Zero For Sulphur.
From www.scribd.com
Standard Enthalpy of Formation PDF Solvation Chemical Process Standard Enthalpy Of Formation Is Zero For Sulphur Elements in their standard state are not. the standard enthalpy of formation of any element in its standard state is zero by definition. by definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions,. for any element at any particular temperature, we define the standard enthalpy. Standard Enthalpy Of Formation Is Zero For Sulphur.